CDMO Services
Flexible and collaborative service
By providing high-quality GMP manufacturing services,
our service breaks through the limits of cell therapy.
Introduction to Consignment Production
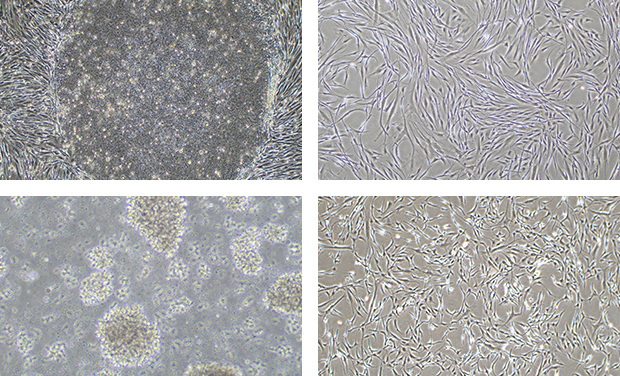
The technical expertise provided
- Consists of a large number of cell therapy specialists with more than 10 years of experience.
- Experts in a variety of cell culture, differentiation, and proliferation techniques.
- Able to perform cell separation and freezing from human-derived tissues.

Production capacity
- Production of medicines for non-clinical trials.
- Production of Investigational Products.

Operates GMP Facility
- Access to independent manufacturing facilities and testing facilities.
- Obtained domestic and international manufacturing licenses.
-
 Advanced Biopharmaceutical
Advanced Biopharmaceutical
Manufacturing License -
 Japan PMDA Overseas
Japan PMDA Overseas
Manufacturing Facility
Recognition Permit -
 Obtained permission for
Obtained permission for
management business
of human cells -
 Cellatoz cell processing
Cellatoz cell processing
facility report approved -
 Pharmaceutical
Pharmaceutical
manufacturing license
Introduction to the testing services
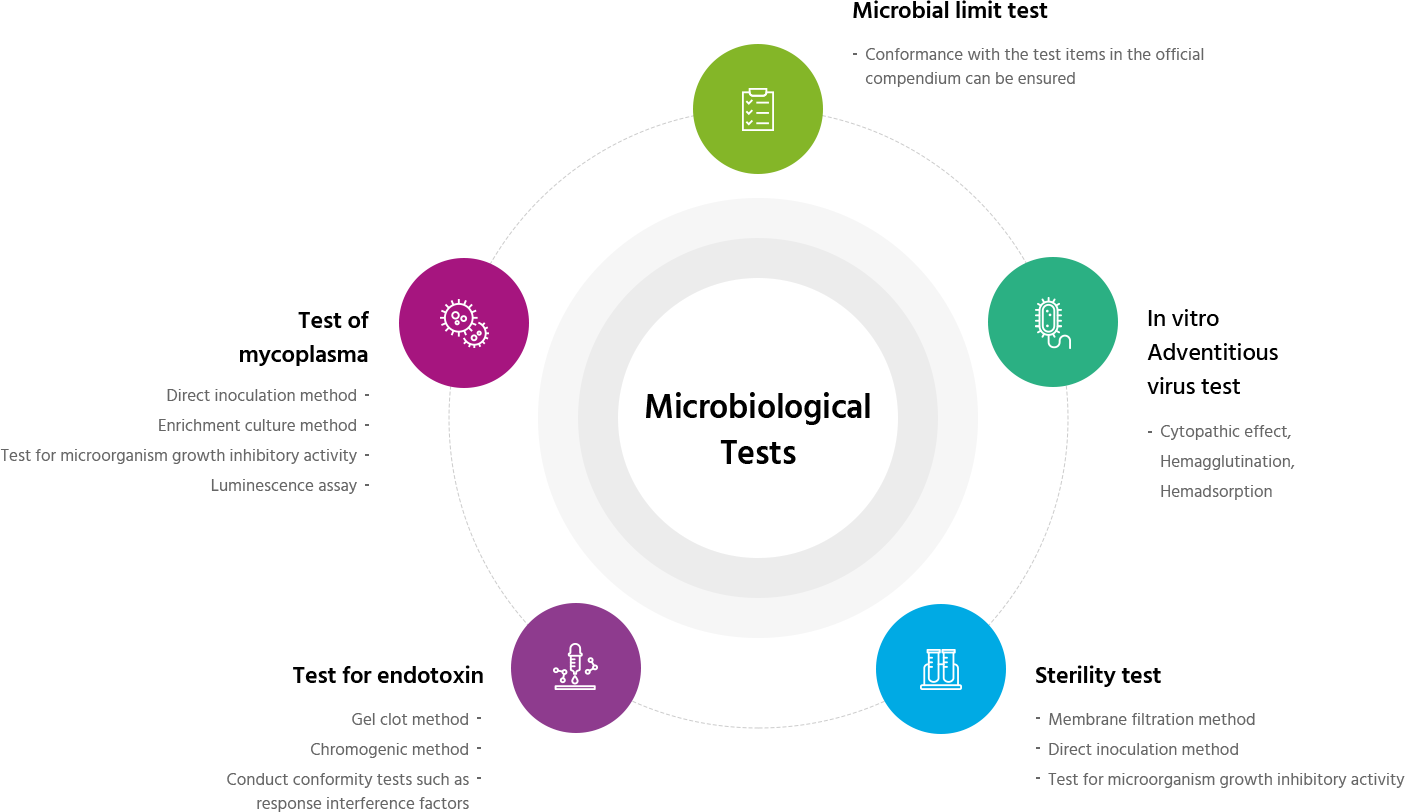
Analytical services
-
 Cell characteristics
Cell characteristics- Cell growth
- Cell differentiation
- Cell phenotype
- Immunogenicity
-
 Stability test
Stability test -
 Leak test
Leak test -
 Foreign insoluble matter test
Foreign insoluble matter test
Facility introduction

GMP 입구

GMP 입구

GMP 입구

일반시험실
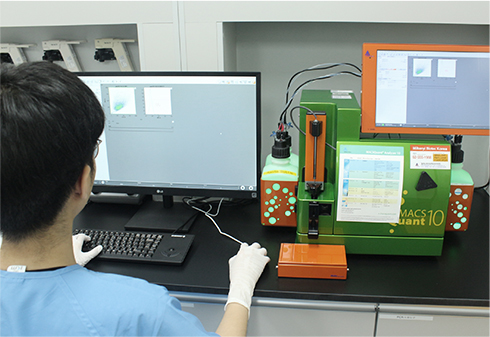
일반시험실
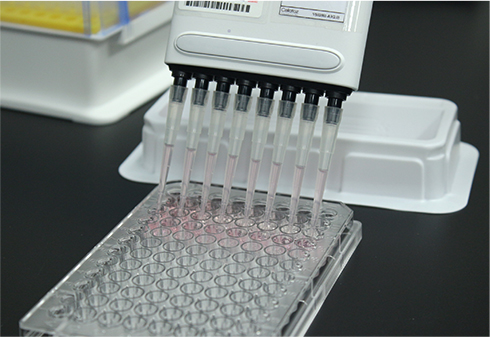
일반시험실

일반시험실

일반시험실

일반시험실

일반시험실

바이러스실

바이러스실

세포보관소

세포보관소

패스박스

제조실

제조실

제조실

제조실

제조실

제조실
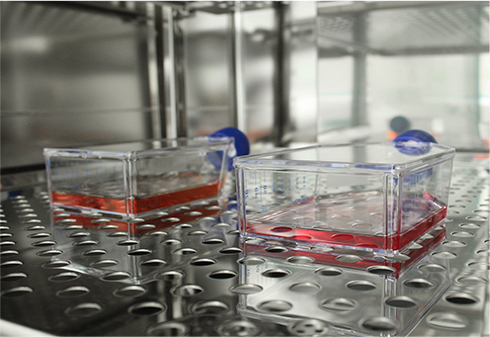
제조실

제조실

제조실

제조실

제조실

제조실

사무실 입구
PROCESS
-
01Pre-contract
-
02Process
Development -
03Technology
Transfer and
Pilot Runs -
04Process Assay
Development -
05Engineering
Runs -
06Aseptic
Process
Validation -
07Process
Validation -
08Production
CONTACT US
 gelim@cellatozrx.com
gelim@cellatozrx.com 070-5165-3974
070-5165-3974
Mon - Fri
8:00 - 17:00
8:00 - 17:00
ABOUT
We have a system that can perform many tasks, from basic research to high-tech biopharmaceutical production.
Download the service policy